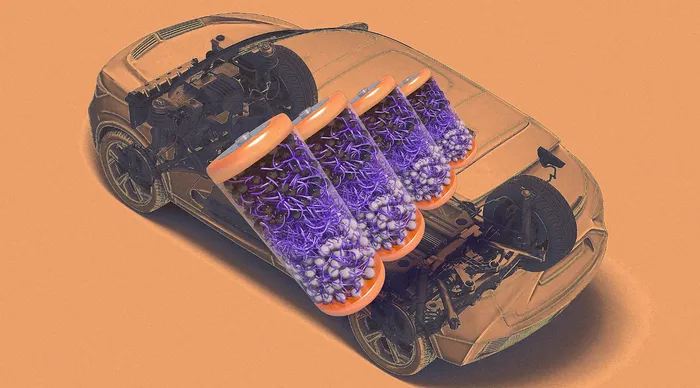
.
Image: Courtesy of the researchers/MIT News, leonello/iStock/Getty Images Plus
This year, global EV sales are expected to jump almost 25% compared with 2024. As the demand for electric vehicles soars, there’s a looming concern for industry experts: figuring out the best way to repurpose the several-hundred-pound batteries that power these vehicles.
According to a 2023 study by McKinsey, the global supply of EV batteries for recycling is steadily increasing and is expected to hit a whopping 7,850 kilotons in 2035. That same year, McKinsey projects that EV battery recycling will be a $7.2 billion industry in the U.S. Currently, though, experts are still trying to find the best way to actually scale the recycling process. The prevailing strategy is a technique that essentially involves shredding EV batteries into a superfine powder—a process that has proved costly, complicated, and inefficient.
Now, researchers at the Massachusetts Institute of Technology have published a study showing a new way to potentially bypass the shredding step altogether. According to Yukio Cho, lead author on the study and a Stanford energy postdoctoral fellow, the team has developed a new way to build a battery that makes it much easier to separate its component parts, leaving them ready for recycling.
The two main ways that EV batteries are diverted away from landfills are through reuse and recycling. Some companies are finding ways to repurpose EV batteries after they’re no longer fit for driving. One startup is using retired EV batteries to power up an entire data centre in Nevada, for example, while another is repurposing old batteries to run new EV charging stations.
Others are searching for ways to break these batteries down and reuse their valuable components. The current industry standard is to shred the batteries into a fine powder called “black mass,” which has to be sorted into salvageable metal parts. The sorting process is messy, complicated, and often requires specialised facilities in advanced recycling markets like China to actually make the metals usable. Even then, Cho says, the acids used to sort out the metals can pose an environmental risk—and, to top it all off, the whole process is expensive.
“Elemental components are so complicated,” Cho says. “Once you’ve generated this black mass, it’s really difficult to make recovering the critical materials cost-positive.”
Cho says there’s not much consensus among experts today on how many EV batteries are actually getting recycled and how many are being diverted to landfills. What is clear, though, is that there’s plenty of motivation to turn EV manufacturing into a more circular economy. To start, siphoning e-waste into trash heaps poses the risk of leaching hazardous materials into soil and water. From an economic perspective, EV batteries also contain valuable metals like nickel, cobalt, manganese, and lithium, which can be harvested and reused to prevent more expensive and polluting ore-mining operations.
To skirt around the issue of black mass entirely, Cho and his team decided to take a totally novel approach to EV battery design.
“So far in the battery industry, we’ve focused on high-performing materials and designs, and only later tried to figure out how to recycle batteries made with complex structures and hard-to-recycle materials,” Cho told MIT News in an interview. “Our approach is to start with easily recyclable materials and figure out how to make them battery-compatible.”
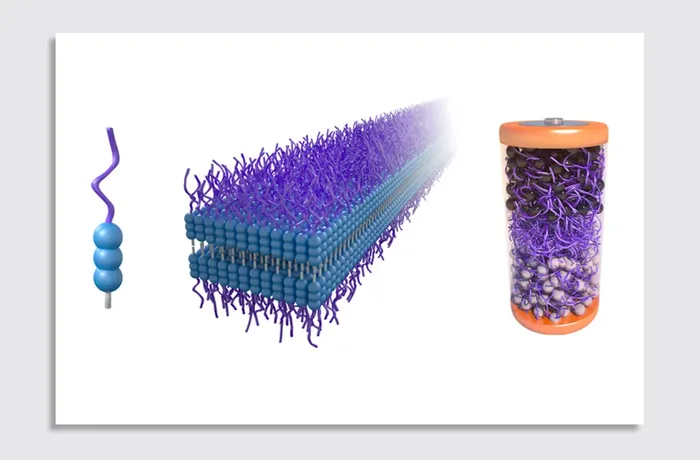
A rendering shows (left) the mPEGAA molecule designed by researchers, (middle) how the molecules self-assemble into nanoribbons, and (right) how the molecules are used for the battery electrolyte.
Image: Courtesy of the researchers
EV batteries are made of three main parts: the positively charged cathode, the negatively charged electrode, and the electrolyte that shuttles lithium ions between them. Typically, EV batteries are sealed so tightly that, in order to take them apart efficiently, shredding them becomes the best way to recycle them. The novel innovation from the MIT team is a new electrolyte material which, when soaked in an organic solvent, “just dissolves like cotton candy,” easily separating the batteries’ parts.
Cho compares the innovation to a hypothetical ham sandwich. Imagine that the sandwich has been glued shut, and in order to retrieve the bread, lettuce, and ham, it has been shredded and must be sorted by minute particles. Now, imagine that the sandwich was held together by mayo instead: You could easily separate all of the sandwiches’ components. That’s essentially the difference between the black mass recycling step and the electrolyte process that his team is working on.
Cho’s team created a solid-state battery to test the material, finding that it held up against the battery’s demands. Then, once the battery was treated with an organic solvent, the material dissolved—cutting out the necessity of a shredding step entirely.

A depiction of batteries made with MIT researchers’ new electrolyte material, which is made from a class of molecules that self-assemble in water, named aramid amphiphiles (AAs), whose chemical structures and stability mimic Kevlar.
Image: Courtesy of the researchers/edited by MIT News
There are a few shortcomings with the current dissolvable prototype. To start, Cho says the test battery’s performance was well below that of today’s gold-standard commercial batteries.
“The performance is at a level that the industry will never think about—if you have an iPhone 13, you’ll never think about swapping that for an iPhone 4,” Cho says. “Matching the performance to the current state-of-the-art batteries is definitely a challenge we haven’t demonstrated yet.”
Part of that performance deficit, Cho says, likely comes from the fact that his team built its battery from the ground up. While it will be at least several years before this new material might be commercially viable, he believes it could be swapped into future EV batteries without too much hassle on manufacturers’ parts.
“I think in the future, we can integrate this material as a part of the battery,” Cho says. “If you imagine that it dissolves like cotton candy, it can just be a very thin layer somewhere in between the component parts. That will serve the purpose of opening the battery in an autonomous way.”
ABOUT THE AUTHOR
Grace Snelling is an editorial assistant for Fast Company with a focus on product design, branding, art, and all things Gen Z. Her stories have included an exploration into the wacky world of Duolingo’s famous mascot, an interview with the New Yorker’s art editor about the scramble to prepare a cover image of Donald Trump post-2024 election, and an analysis of how the pineapple became the ultimate sex symbol
FAST COMPANY
